 MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia | European Respiratory Society
MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia | European Respiratory SocietyMain menuUser menuBúsquedaMRSA as a cause of lung infection, including air infection, community-acquired pneumonia and hospital-acquired pneumoniaAstractionThe pneumonia acquired by the community has been recognized as a cause of community-acquired pneumonia, although rare, and a major cause of health-related pneumonia (HA). The resistance of S. aureus to methicillin developed shortly after its introduction into clinical practice. Since then, S. aureus (MRSA) resistant to methicillin has been predominantly a feature of infections acquired by the hospital, or finally HA, since the boundaries have become more blurred between the community and the hospital environments. However, more recent infections have been detected acquired by the community (CA)-MRSA and are increasingly common, especially in the US. Europe has not been immune to the development of MRSA in health settings and although the prevalence of CA-MRSA is currently relatively low, there is a risk of increased spread. These new CA-MRSA strains seem to behave differently to HA-MRSA strains. Although they predominantly cause skin infections and soft tissue, mainly as cooking and abscesses that require drainage, invasive infections that threaten life, including necrosant pneumonia, can also occur. This article summarizes pathogenesis and clinical presentations of MRSA-related lung infections. SERIE "MRSA AND THE PULMONOLOGIST" Edited by M. Woodhead and A. Torres Number 2 in this series Staphylococci are spherical, gram-positive bacteria that occur in microscopic clusters that resemble grapes. Staphylococcus aureus mainly colonizes nasal passages, but can be found regularly in most other anatomical sites. Adult transport rates vary from 20 to 50% with people who are persistent carriers, intermittent carriers or noncarriers. A large study found that 24 per cent of people persistently carry S. aureus and 57 per cent are intermittent carriers while 20 per cent were never colonized. S. aureus causes a number of important infections both in the community and in the health environment. These include skin and soft tissue infections, bone and joint infections, bacteremia, endocarditis and pneumonia. It is also important in a variety of toxins, such as food poisoning by superantigenic enterotoxin (SAE), scaling skin syndrome in neonates by exfoliant toxin and toxic shock syndrome by toxin from toxic shock syndrome (TSST). S. aureus has a large repertoire of virulence factors, including structural products and secrets that play a role in pathogenia (Fig. 1). Some of the determinants of virulence of Staphylococcus aureus. TSST: Toxin from toxic shock syndrome. Examples of S. aureus viruence factors include the following. (1) Surface proteins, for example protein A, that promote adherence and therefore colonization of host tissues. Different S. aureus strains may have different groups of these proteins predisposing different types of infections. 2) Invasions that promote bacterial propagation in tissues (leucocidine, kinase and hyaluronidase). 3) Membrane harmful toxins that smooth the eukaryotic membranes (haemolysins, leucootoxin and leucocidine). 4) Exotoxins that damage the host tissues or otherwise cause symptoms of disease (SAE-G, TSST-1, toxin exfoliatin and Panton-Valentine leukocidin (PVL)). (5) Hereditary resistance and acquired to antimicrobial therapeutic agents. The expression of these virulence factors is influenced by different environmental signals, and there are a number of regulatory genes directly involved in their control, such as agr, spa, sar and sigB, . The regulatory locus of the accessory gene, agr, regulates a range of genes including haemolysins α, β, γ and δ, as well as leucocidina and PVL. Controls of spa regulator genes for protein A synthesis. Therefore, different strains of S. aureus may contain different adhesins or toxins, or differ in their ability to form biofilm. In lung infections, several models of mouse pneumonia have shown the importance of protein A, α-haemolysin and PVL in pneumonia pathogenesis. The model of mouse pneumonia by Labandeira-Rey et al. compared the positive PVL strains of Staphylococcus aureus resistant to methicillin (MRSA) to negative PVL strains that show that the former caused necrotissant pneumonia similar to human sight, while PVL-negative strains did not. However, the precise role of PVL in lung infections has not been fully explained. In a different model of pneumonia mouse, Bubeck Wardenburg et al. found that agr and spa mutants could not cause lethal lung infections that increase the possibility that α-haemolysin and protein A are important in lung parenchyma damage. In fact, immunization against α-haemolysin appears to protect mice from lethal pneumonia and can provide an early insight into possible future vaccine strategies. It is likely that a combination of many factors will cause improved virulence of certain strains of S. aureus. Staphylococci has developed resistance to methicillin by various mechanisms. The characteristic mechanism of MRSA is the acquisition of the mecA gene, probably through a mobile genetic element, known as the mec of chromosome of cassette staphylococcal (SCCmec), of coagulase-negative staphylococci. At least five types of the SCCmec genetic element (SCCmec I-V) have been identified. MecA gene codes for a penicillin binding protein variant with a low affinity for β-lactam antibiotics, thus effectively reducing the activity of these drugs. Initially, MRSA was associated exclusively with the acquisition of hospitals and other health environments. Apparently, the acquired community (CA)-MRSA, described in the 1980s and 1990s could be generally linked to health adjustments. This led to a new terminology of the MRSA "associated by health care" (HA)-MRSA and "associated by the community." The HA-MRSA and MRSA strains associated with the community were very similar in terms of molecular typography and antimicrobial susceptibility, with both partners with SCCmec I-III resistance elements against many kinds of antibiotics – however, at the end of the 1990s CA-MRSA was identified as the cause of serious and fatal infections occurring in previously healthy groups of children in North America. The cases of CA-MRSA that cause skin infections and soft tissue and necrotisant pneumonia have been widely reported in other healthy individuals . CA-MRSA, unlike HA-MRSA, is associated with SCCmec IV and V that, in addition to the mecA gene, may contain encoder genes for toxin production –. One such toxin, the PVL toxin, destroys leukocytes and causes an extensive tissue necrosis, thus having a clear role in necrotissant pneumonia pathogenesis, . As previously said, it is unlikely that PVL only represents the greatest invasiveness of CA-MRSA, rather than a combination of virulent factors, including Aγly protein The principle differences between CA-MRSA and HA-MRSA are summarized in table 1 . Epidemiology and the clinical burden of MRSA infections in Europe will be discussed later. Typical differences between HA-MRSA and CA-MRSAMRSA and LUNG The effect of S. aureus in the airways, from asymptomatic colonization to severe pneumonia, depends on the interaction of patient, environmental and bacterial factors. The colonization of the lower respiratory tract by S. aureus and therefore MRSA may occur in the establishment of a chronic lung disease, such as chronic obstructive pulmonary disease and supurative lung disease, or due to violations in natural defenses, such as endotracheal intubation. Although this colonization can be asymptomatic, it paves the way for over-infection, i.e. pneumonia, if the balance between the amphibian defense and the bacterial virulence moves in favor of the bacteria. MRSA pneumonia can also occur in previously healthy patients without risk factors for colonization. The pneumonia acquired in the hospital (HAP) or the nosocomial pneumonia is generally defined as pneumonia that develops ≥48 h after admission to the hospital that did not incubate at the time of admission. Ventilator-associated pneumonia (VAP) is usually defined as pneumonia that develops ≥48 h after the implementation of endotracheal intubation and/or mechanical ventilation and was not present before intubation . VAP can be divided in early and late start. Early startup disease occurs within 4-5 days of admission and tends to be caused by community-type antibiotic-susceptible pathogens, while late startup disease tends to be caused by antibiotic-resistant pathogens. However, some studies have found an increasing frequency of early-initiated PH caused by pathogens more commonly associated with nosocomial disease. This has contributed to the concept of health-related pneumonia (HCAP), which involves pathogens associated with recent prior hospitalization and/or antimicrobial therapy. HCAP has been defined as a pneumonia that occurs in any patient who has: been admitted to a acute care hospital for ≥2 days within 90 days of the infection; was resident in a nursing home or long-term care center (LTCF); attended a hospital or hemodialysis clinic; or received recent intravenous antibiotic therapy, chemotherapy or wound care within 30 days of the current infection. A subpopulation of these patients is those with pneumonia acquired in the nursing home. While the MRSA is considered by many in North America and elsewhere, as an important pathogen in the nursing/LTCF environment, Europe's data are not consistent with this and an additional assessment is needed. In this context, the presentation of pneumonia is clinically similar to that caused by gram-negative organisms, and has an associated mortality of all causes of 55.5 per cent, regardless of appropriate early therapy. In the European environment, S. aureus remains an unusual primary cause of community-acquired pneumonia (CAP), although it is an important cause of pneumonia and death after flu. The role of CA-MRSA is even more ill-defined, though emerging in the UK and Europe. CA-MRSA infections have a symptom start before or within 48 hours of hospital admission and patients do not have significant prior medical contact. The CAP, which is due to CA-MRSA, presents classicly in a young, previously healthy individual with progressive and severe rapid respiratory disease. The aggressive nature of CA-MRSA, due to the production of toxin, causes massive destruction in the previously normal lungs. PNEUMONIA: CLINICASCAPStaphylococcal pneumonia has been a changing clinical entity since it was initially reported in the late nineteenth and early twentieth century. He was recognized in young and healthy military personnel during the First World War as a post-influenza pneumonia with a quick start of symptoms. Case descriptions highlighted the presence of isolated tachypnoea without other signs of severe disease and normal chest X-ray (CXR) in the first 24-48 h. Death occurred quickly and occurred in 80–90% of cases in this pre-antibiotic era. Post mortem findings revealed lung hemorrhage and microabsces formation, . If patients survived until day 4-5, clinical signs of bronchiomony were developed and CXR revealed cavities, empyema and piopneumothoraces. In the 1950s, cases of estrofacca pneumonia began to be reported in people without any previous flu infection. They usually had some predisposed risk factors, such as cardiopulmonary disease, alcoholism or diabetes mellitus, or had acquired an infection in the hospital. These strains included methicillin-sensitive strains, as well as MRSA. The clinical presentation was generally less explosive than had been described above, and mortality in the antibiotic era varied from 20% in young adults to 30–50% in post-influenza cases to 83% in patients with primary bactericiaemic pulmonary pneumonia – in addition to primary pneumonia resulting from direct inoculation of the lungs, estrocodic pneumonia can also be developed by hematogenopathy. HAP and VAPUntil recently S. aureus represented for √1–5% of CAP cases and ±10–15% of PAH cases, but in the last 10–20 yrs there have been significant changes in Staphylococcal pneumonia epidemiology. First, there has been a dramatic increase in the proportion of S. aureus infections due to MRSA, which is now responsible for √50% of all S. aureus infections in some intensive care units (IUs). At the same time, the ventilation support of an aged population has increased, which often has a significant comorbidity. Together, this has led to an increase in MRSA pneumonia, with 20-40% of all cases of HAP in the US, including VAP, due to MRSA. VAP due to MRSA is associated with a worse and greater use of resources than VAP due to other agencies. It is estimated that PAH occurs at the rate of 4 to 50 cases per 1,000 admissions to community hospitals and general medical rooms, and 120 to 220 cases per 1,000 ICU admissions. It has a percentage of antibiotics prescribed in ICU, and has an associated attributable mortality of 33–50%. PAH requires the entry of microbial pathogens in the lower respiratory tract followed by colonization which, if the body's defenses are overwhelmed, leads to excessive infection. Factors such as the severity of the underlying disease of the patient, previous surgery, antibiotic exposure, other medicines, and exposure to invasive respiratory devices and equipment are important in the pathogenesis of HAP and VAP. Starting time, relative to hospital admission, HAP and VAP, is an important risk factor for the patient's outcome and for specific pathogens. Early start disease, defined as within 4 days of hospitalization, has a better prognosis and is more likely to be caused by antibiotic-sensitive bacteria. Exceptions to this are patients with recent pre-hospitalization and/or antibiotic use and older persons residing in LTCFs. These patients with HCAP have a spectrum of pathogens that closely resemble HAP and late-initiated VAP with up to 33% of being due to MRSA . VAP due to MRSA seems to have a significant excess of morbidity and mortality regardless of the proper antimicrobial treatment and patient characteristics. Compared to ICU controls, an estimated over-mortality of 22.7 per cent was observed in Spanish ICU patients with MRSA VAP; this was despite proper glycopeptide therapy. In the same study, the cases with MRSA VAP also had a longer length of stay in the ICU compared to the controls (about 33 and 21 days respectively) , . A prospective study comparing the result of PAV by causative pathogen showed that in patients who received appropriate initial antimicrobial therapy, cases due to MRSA still had a significantly slower clinical resolution than those due to other pathogens. Fever and hypoxia resolution within 72 h occurred in only 30% of cases of MRSA VAP, compared to 93.3% of cases S. aureus sensitive to meticine (MSSA), 100% due to H. influenzae and 73% due to Pseudomonas aeruginosa. The clinical resolution and ventilation unit in patients with properly treated MRSA VAP was similar to that of patients with VAP due to aeruginosa who received inappropriate antibiotic therapy. A new form of CAPThere has been a rise of CA-MRSA CAP that is reported on both sides of the Atlantic. Although CA-MRSA is primarily a cause of skin infections and soft tissue, it can also cause severe necrotising pneumonia, –. Some of these respiratory infections have been associated with septic shock, haemoptissis, respiratory insufficiency and intake of intensive care for ventilatory or circulatory support. These infections in previously healthy young patients resemble those reported in the early twentieth century. The CA-MRSA CAP features often occur in young adults previously healthy, up to 75% of cases, with a previous flu-like disease, . Painters quickly develop severe respiratory symptoms, often including hemooptisis, hypotension, and high fever. Characteristically, leucopenia is produced and C-reactive protein is elevated (with 350 g·L−1). CXR findings of multi-lobar cavitation alveolar infiltration are also consistent with CA-MRSA, , . These features are not specific to CA-MRSA, but are consistent with PVL producing S. aureus and therefore may be applicable to some MSSA strains. However, the young age at CA-MRSA CAP has been a finding consisting of studies in Europe and the US, . Table 2 summarizes when it is suspected that CA-MRSA in CAP and Table 3 summarizes the risk groups with the highest rates of CA-MRSA colonization. Summary of when to suspect CA-MRSA in community-acquired pneumonia Risk groups with growing colonization rates CA-MRSA INVESTIGATIONS FOR STAPHYLOCOCCAL PNEUMONIA Adrenal cultures should not be used to diagnose VAP as broncoalveolar washing specimens are preferred. For HAP, the less costly, less invasive and faster sampling technique that requires a minimal experience should be used to establish microbiological diagnosis, for example, undirected bronchoalveolar (heaven) not bronchoscopy, blood cultures are more likely to be positive in secondary pneumonia where the primary source is elsewhere, such as infective endocarditis or primary discneony. Blood cultures are also more likely to be positive in the PAV than PAH, 24–36% compared to 5–15%, respectively. Therefore, because the blood cultures are often negative, it is important to obtain an adequate specimen of the respiratory tract before initiating therapy. Appropriate specimens include endotracheal sampling or pleural but not skeptical fluid, as S. aureus is frequently present in the upper respiratory secretions of healthy individuals. Antibiogram: Antibiotic Sensitivities After isolating S. aureus, sensitivity tests for several antibiotics will determine whether it is MRSA or not, and what antibiotics can be clinically effective. Until the appearance of CA-MRSA, the insulations of MRSA (HA-MRSA) were not only resistant to methicillin and therefore resistant to all β-lactams, but were often multiresistant and resistant to a range of other antibiotic groups. However, the CA-MRSA antibiogram is commonly only resistant to β-lactams and susceptible to most other antibiotic classes. This difference in laboratory findings can provide a clue that the patient has a CA-MRSA insulation instead of a HA-MRSA insulation. However, over time, CA-MRSA is likely to acquire the resistance genes that will make it harder to differentiate from HA-MRSA through routine antimicrobial susceptibility tests. Molecular Techniques New laboratory techniques, including microarrayas to detect PVL and possibly other staphylococcal or superantigenous toxins, can help in the diagnosis of CA-MRSA pneumonia. These micro-roots can reveal whether isolates are hosting the genes for several toxins including PVL and leucocidine, which have been linked to lung disease and have been associated with CA-MRSA isolates. Also in research and development, rapid molecular-based tests are performed to detect PVL, mecA and type IV SCCmec. There are no very specific radiological characteristics for Staphylococcal pneumonia. Early in progression of CAP disease with S. aureus may have infiltrated minimal but progress quickly, even within hours. There may be unilateral or bilateral consolidation, especially in the PVL producing CA-MRSA. Compared to the HA-MRSA pneumonia these infiltrates are more prone to cavitar, which can be seen in series CXR and better confirmed by a CT scan analysis, . Pleural effusions, pneumatoceles and pneumothoras are also common findings. However, for HAP and VAP there are no radiological features that distinguish MRSA from any other causating organism and, in addition, VAP can be lost in CXR up to 26% of the cases. However, clinically there may be suspicion of MRSA as a causative organism in VAP, as patients tend to have a more severe disease and respond more slowly to appropriate antimicrobial therapy. Consequently, radiological progression can be faster than with other organisms (fig. 2). Necrotizing pneumonia in (a) a chest X-ray and (b) a CT scan obtained on day 3. Computed tomography shows multiple nodullar lesions and bilateral cavities. Reproduced with permission from the editor. CONCLUSIONS The frequency of pneumonia caused by HA-MRSA and CA-MRSA is increasing. HA-MRSA is traditionally present in the hospital setting, although it has been suggested that many older patients, who often have comorbidities and who reside in the LTCFs in the community, may acquire respiratory infections caused by pathogens more traditionally found in the hospital or in the health environment. The evidence that this is the case in Europe is unequivocal and requires more research. A new form of MRSA, more virulent and often more toxin it produces (including PVL toxin), is coming out of the community. It is mainly affecting healthy young people and has a high mortality rate. In light of this, a strong clinical suspicion is needed to instigate appropriate therapy. Vancomycin has been disappointing in treating MRSA pneumonia and although linzolid may be a better option, more data is required to support this as a standard of care. Combination therapy with clindamycin and immunoglobulin can be useful in cases of CA-MRSA where PVL production is causing hemorrhagic and necrotizing pneumonia. Statement of interest A statement of interest to D. Nathwani can be found in footnotes Previous articles in this series: No. 1: Pantosti A, Venditti M. What is MRSA? Eur Respir J 2009; 34: 1190–1196.References Thank you for your interest in spreading the word about European Respiratory Society. NOTE: We only request your email address so that the person you are recommending the page knows that you wanted to see it, and that it is not junk mail. We don't capture any email address. FormatsJump ToMore in this section of TOC Related articlesNavigate About ERJEuropean publications of the Respiratory Society HelpFor authors For readers SubscribeContact usThe European Respiratory Society442 Glossop RoadSheffield S10 2PXUnited KingdomTel: +44 114 2672860Email: journals@ersnet.orgISSNPrint ISSN: 0303-1936 ISSN online: 1399-3003 Copyright © 2021 by the European Society of Respiration
Accessibility links Search modesSearch ResultsStaphylococcus aureusA Time Course for Susceptibility to Staphylococcus aureus ...Messuring Staphylococcus aureus - WikipediaStaphylococcus aureus - WikipediaSevere Methicillin-Resistant Staphylococcus aureusPVL-positive Stausphylococcus (PVL-SA) information ...Several infection with seasonal flu A (H3N2) Virus ...Staphylococcus aureusStaphylococcus aureus ← Johns Hopkins ABX GuideStaphylococcus aureus - Horse - VetbookPage navigation1 Foot links

MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia | European Respiratory Society
Linezolid Attenuates Lethal Lung Damage during Postinfluenza Methicillin-Resistant Staphylococcus aureus Pneumonia | Infection and Immunity
CP and CP-PGN prevented local MRSA infection from progressing to a... | Download Scientific Diagram
Infection Staphylococcus aureus Secondary Methicillin-Resistant Clearance and Enhances Susceptibility to Dependent Phagocytic Ba
MRSA Necrotizing Pneumonia and Peripheral Septic Thrombophlebitis | Consultant360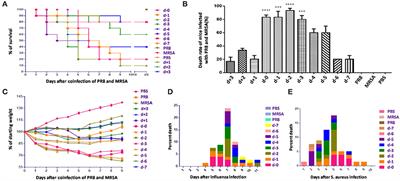
Frontiers | Severe Pneumonia Caused by Coinfection With Influenza Virus Followed by Methicillin-Resistant Staphylococcus aureus Induces Higher Mortality in Mice | Immunology
Virus-Like Particle-Induced Protection Against MRSA Pneumonia Is Dependent on IL-13 and Enhancement of Phagocyte Function - ScienceDirect
Linezolid Attenuates Lethal Lung Damage during Postinfluenza Methicillin-Resistant Staphylococcus aureus Pneumonia | Infection and Immunity
Factors Predicting and Reducing Mortality in Patients with Invasive Staphylococcus aureus Disease in a Developing Country
Predictors of Mortality in Staphylococcus aureus Bacteremia | Clinical Microbiology Reviews
Control of Methicillin-Resistant Staphylococcus aureus Pneumonia Utilizing TLR2 Agonist Pam3CSK4
The first report of Methicillin-resistant Staphylococcus aureus (MRSA) in cystic fibrosis (CF) patients in Saudi Arabia - ScienceDirect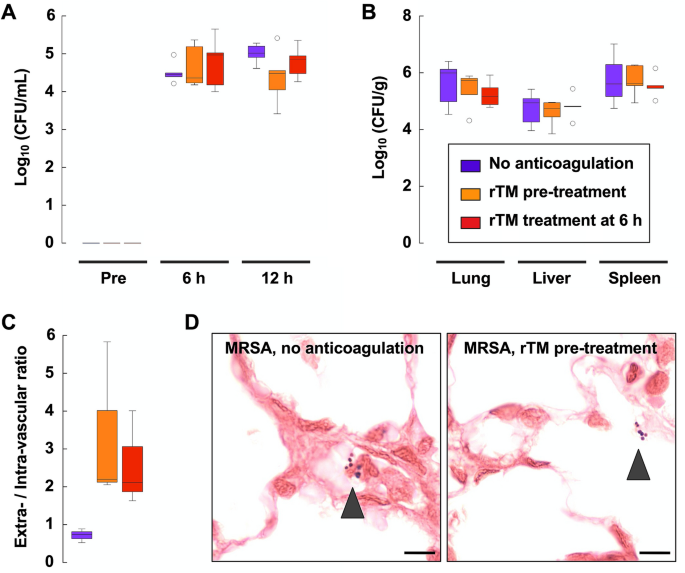
Recombinant thrombomodulin alleviates oxidative stress without compromising host resistance to infection in rats infected with methicillin-resistant Staphylococcus aureus | Scientific Reports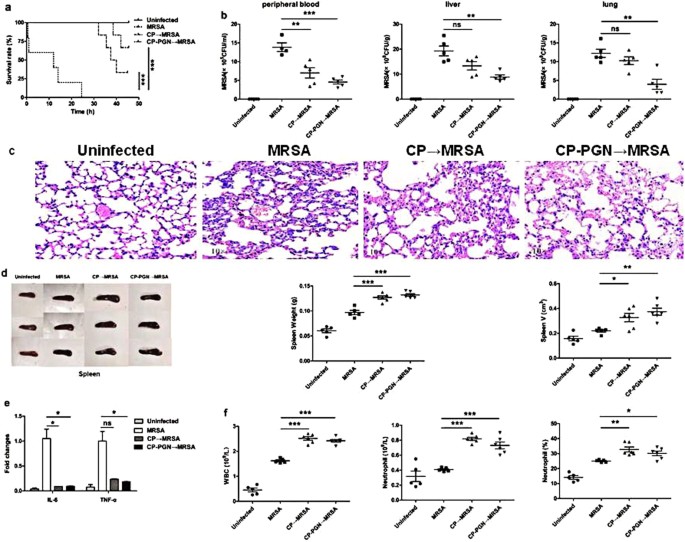
CP and CP-PGN protect mice against MRSA infection by inducing M1 macrophages | Scientific Reports
Advances in the Prevention and Treatment of MRSA in Patients with Cystic Fibrosis – Consult QD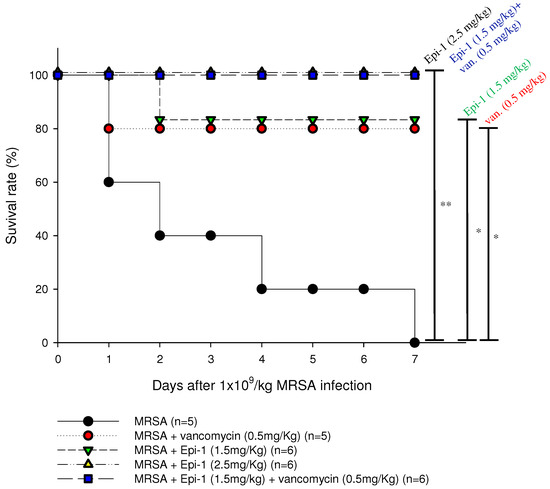
Marine Drugs | Free Full-Text | Epinecidin-1 Protects against Methicillin Resistant Staphylococcus aureus Infection and Sepsis in Pyemia Pigs | HTML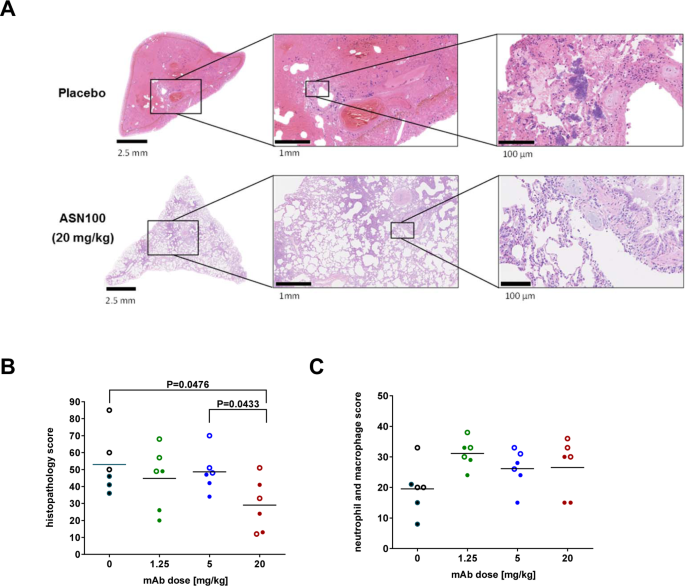
Preventing lung pathology and mortality in rabbit Staphylococcus aureus pneumonia models with cytotoxin-neutralizing monoclonal IgGs penetrating the epithelial lining fluid | Scientific Reports
Efficacy of Azithromycin in a Mouse Pneumonia Model against Hospital-Acquired Methicillin-Resistant Staphylococcus aureus | Antimicrobial Agents and Chemotherapy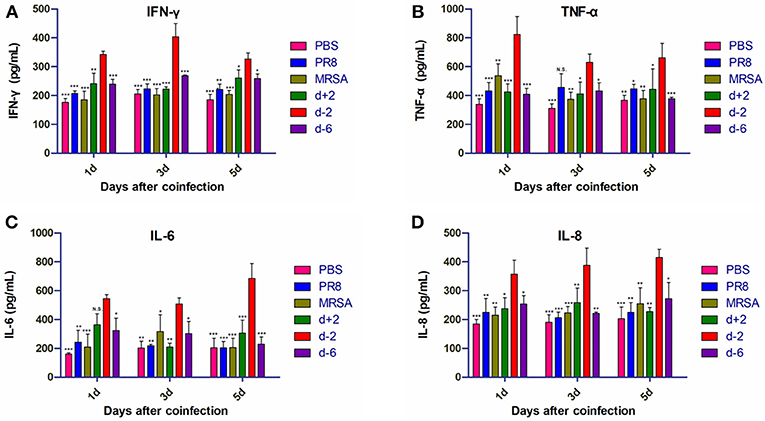
Frontiers | Severe Pneumonia Caused by Coinfection With Influenza Virus Followed by Methicillin-Resistant Staphylococcus aureus Induces Higher Mortality in Mice | Immunology
IVIG-mediated protection against necrotizing pneumonia caused by MRSA | Science Translational Medicine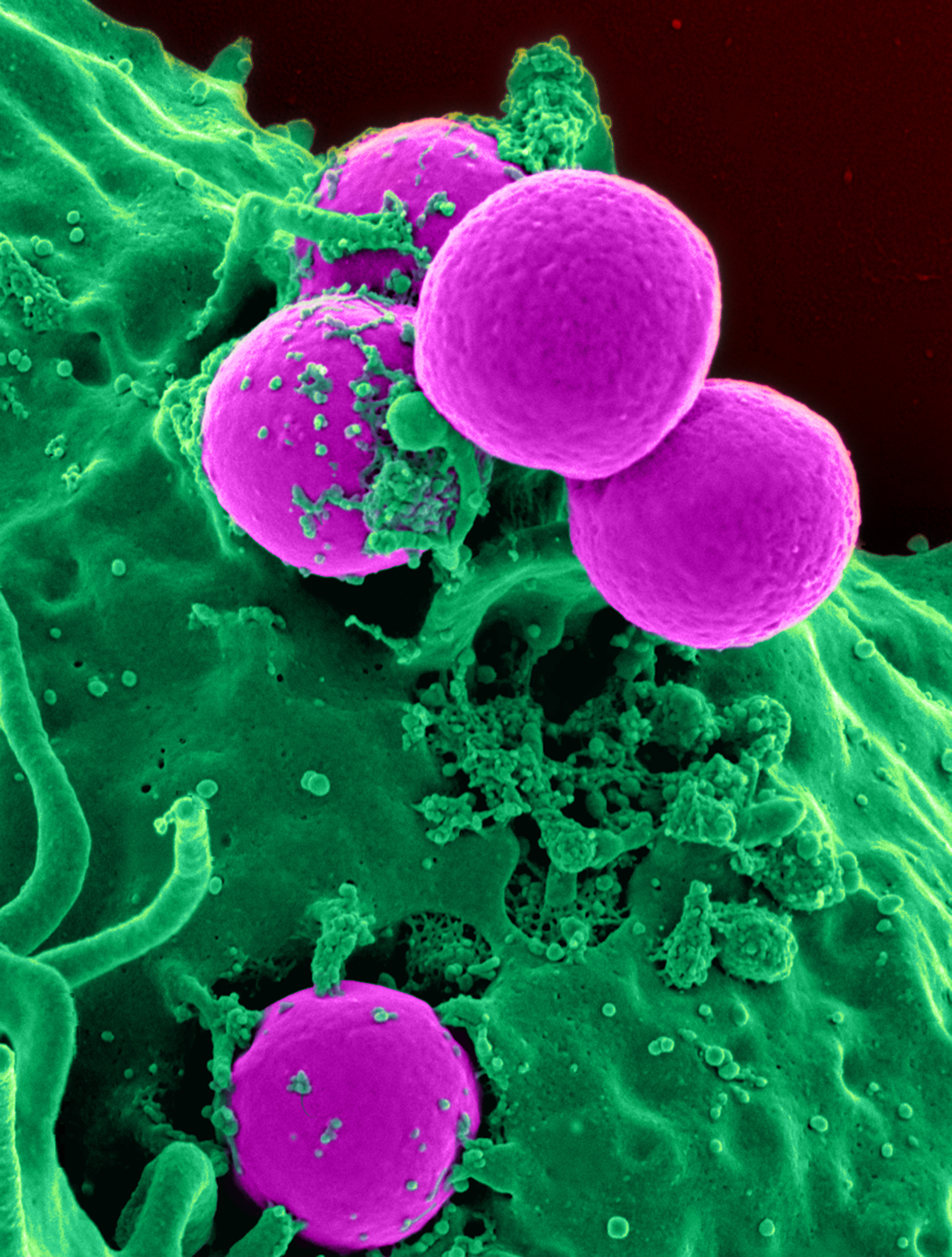
Methicillin-resistant Staphylococcus aureus - Wikipedia
MRSA symptoms: What they are, causes, treatment, and more
MRSA eradication of newly acquired lower respiratory tract infection in cystic fibrosis | European Respiratory Society
CP or CP-PGN combined with vancomycin could improve the survival rate... | Download Scientific Diagram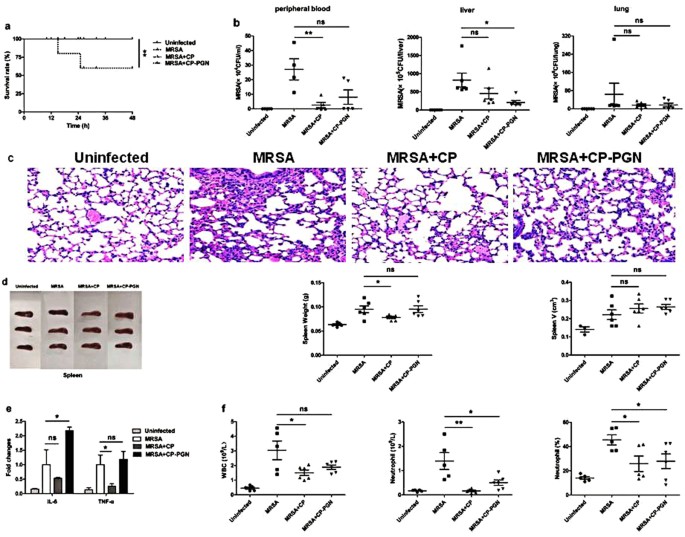
CP and CP-PGN protect mice against MRSA infection by inducing M1 macrophages | Scientific Reports
MRSA: Treatment, causes, and symptoms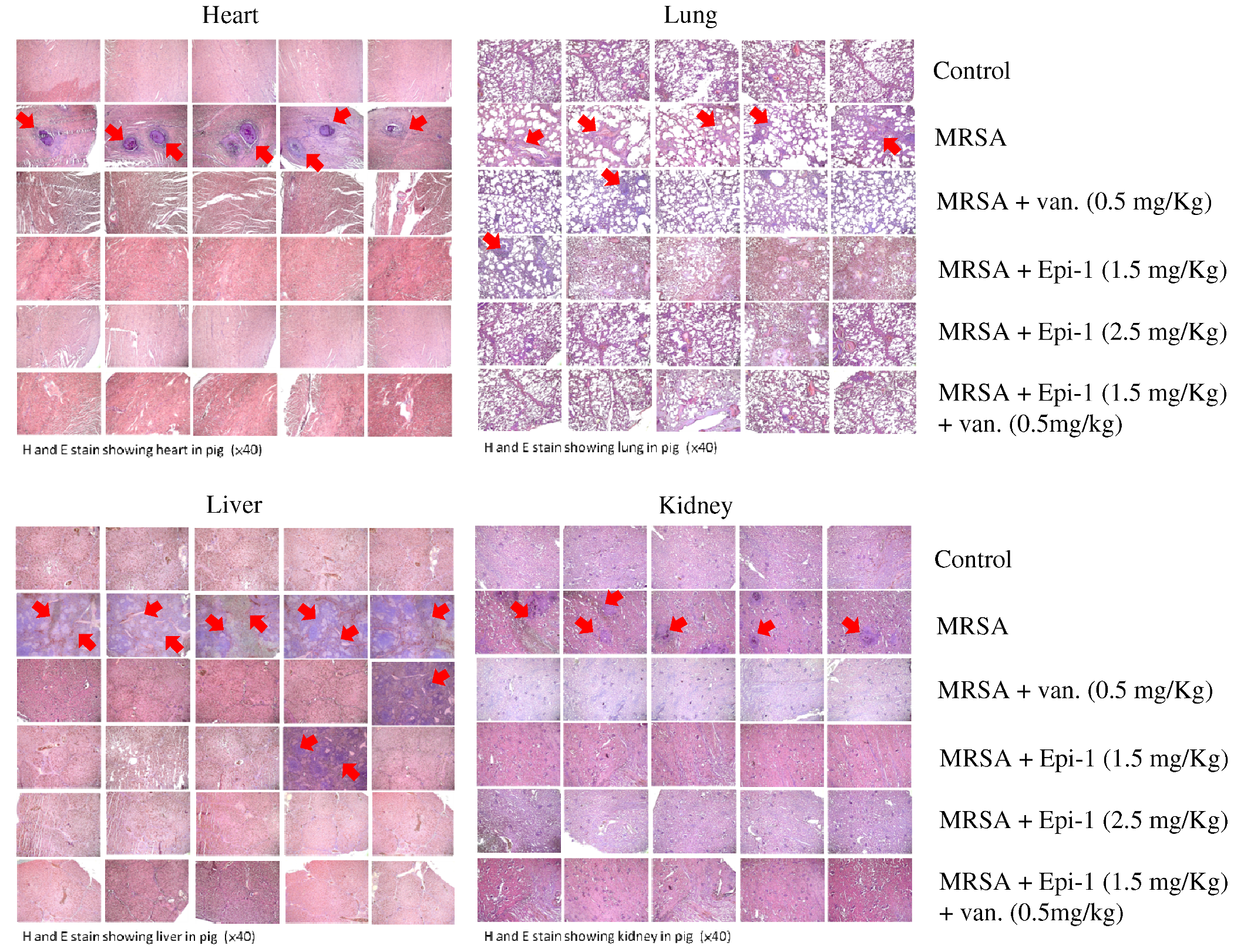
Marine Drugs | Free Full-Text | Epinecidin-1 Protects against Methicillin Resistant Staphylococcus aureus Infection and Sepsis in Pyemia Pigs | HTML
Efficacy of Azithromycin in a Mouse Pneumonia Model against Hospital-Acquired Methicillin-Resistant Staphylococcus aureus | Antimicrobial Agents and Chemotherapy
Linezolid Has Unique Immunomodulatory Effects in Post-Influenza Community Acquired MRSA Pneumonia
MRSA Necrotizing Pneumonia and Peripheral Septic Thrombophlebitis | Consultant360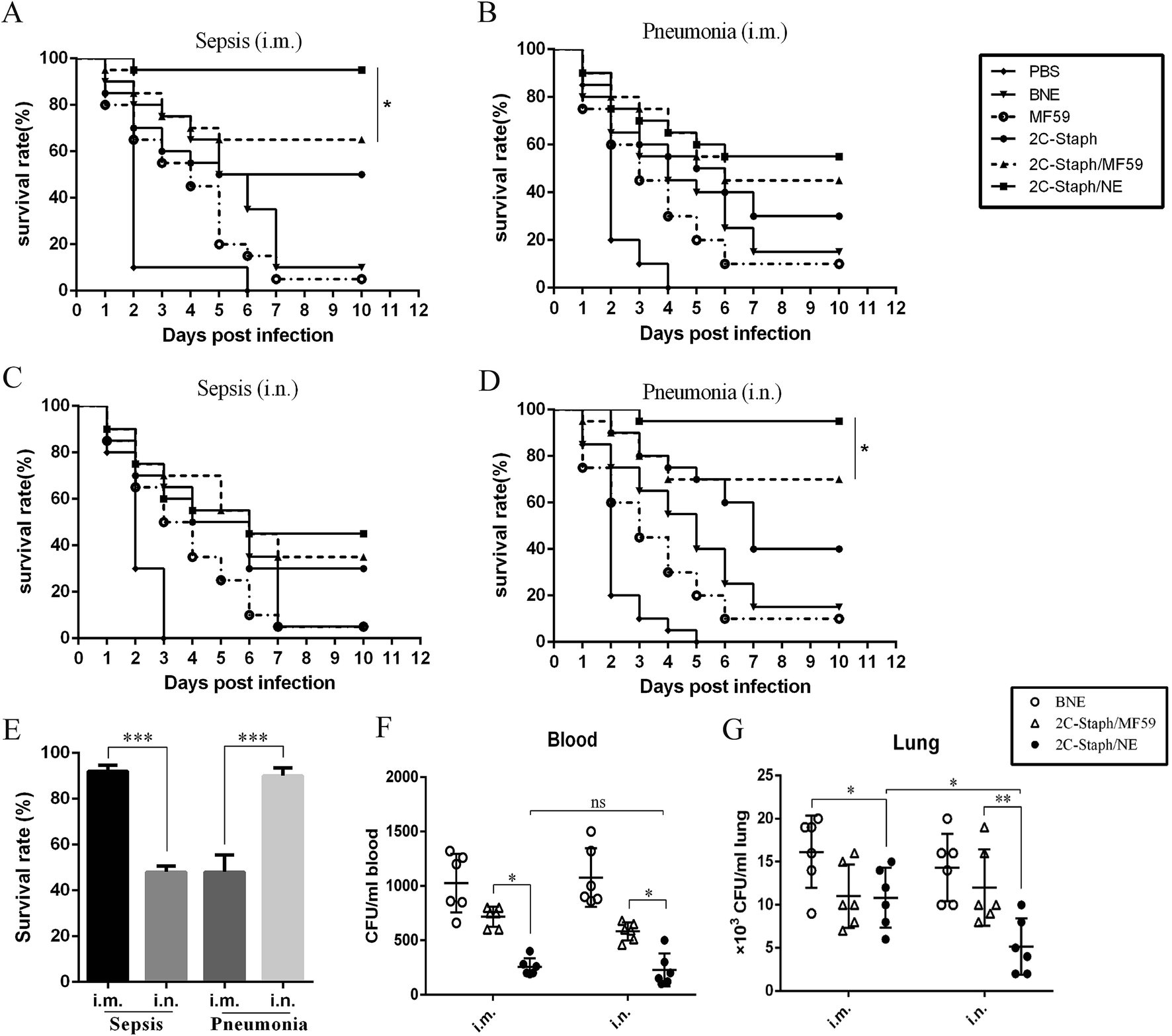
Protective effects of a nanoemulsion adjuvant vaccine (2C-Staph/NE) administered intranasally against invasive Staphylococcus aureus pneumonia - RSC Advances (RSC Publishing) DOI:10.1039/C7RA13630G
Role of MRSA Swabs for De-escalation of Antibiotics in HCAP - ppt video online download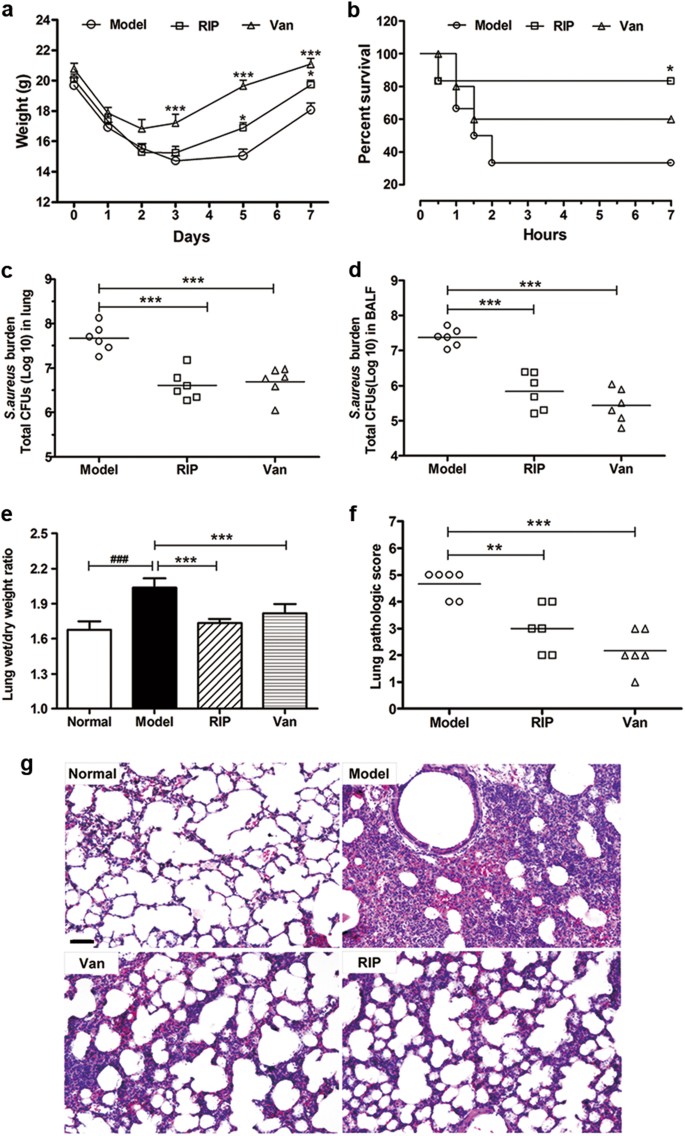
Inhibiting PSMα-induced neutrophil necroptosis protects mice with MRSA pneumonia by blocking the agr system | Cell Death & Disease
What is MRSA in the Sputum? (with pictures)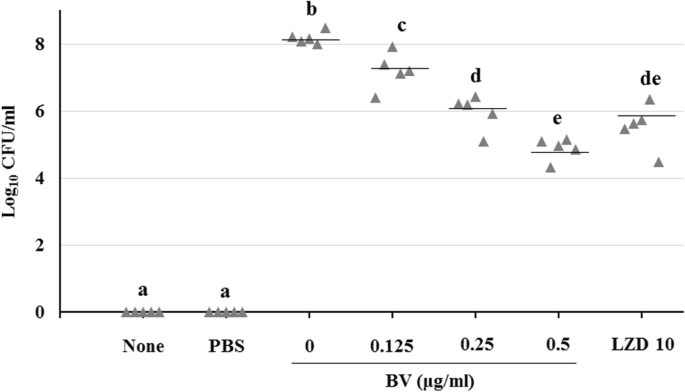
The antibacterial activity and toxin production control of bee venom in mouse MRSA pneumonia model | BMC Complementary Medicine and Therapies | Full Text
Metabolic Adaptation in Methicillin-Resistant Staphylococcus aureus Pneumonia
Influenza Infection Suppresses NADPH Oxidase–Dependent Phagocytic Bacterial Clearance and Enhances Susceptibility to Secondary Methicillin-Resistant Staphylococcus aureus Infection | The Journal of Immunology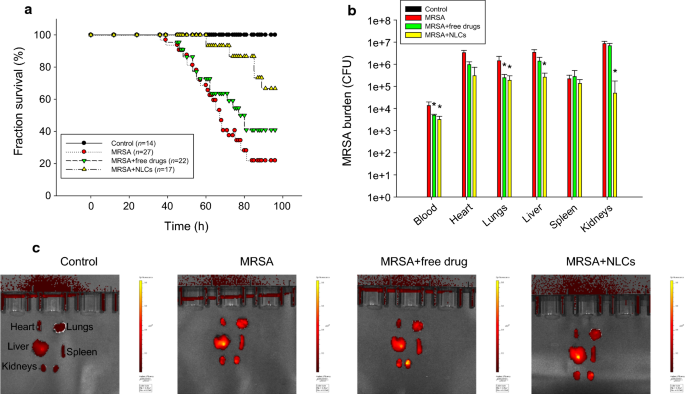
Multifunctional lipid-based nanocarriers with antibacterial and anti‐inflammatory activities for treating MRSA bacteremia in mice | Journal of Nanobiotechnology | Full Text
Vancomycin Monotherapy May Be Insufficient to Treat Methicillin-resistant Staphylococcus aureus Coinfection in Children With Inf
IVIG-mediated protection against necrotizing pneumonia caused by MRSA | Science Translational Medicine
 MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia | European Respiratory Society
MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia | European Respiratory Society

































Posting Komentar untuk "survival rate of mrsa in lungs"