salt tablets for hyponatremia
 Amazon.com: Sodium Chloride Tablets 1 Gm, USP Normal Salt Tablets - 100 Tablets: Health & Personal Care
Amazon.com: Sodium Chloride Tablets 1 Gm, USP Normal Salt Tablets - 100 Tablets: Health & Personal CareMain menu User menuBúsquedaMinimum chronic throbbing hyponatremia: significance and management Abstract The mild chronic hyponatremia, defined by a persistent plasma sodium concentration (72-hour concentration) between 125 and 135 mEq/L without apparent symptoms, is common in outpatients and is generally perceived as inconsecuencial. The association between increased mortality and hyponatremia in patients hospitalized in various environments and etiologies is widely recognized. This review analyzes the importance of mild chronic hyponatremia in outpatient subjects and their effects on mortality and morbidity. It refers to whether this disorder should even be treated and if so, which patients will likely benefit from treatment. The approaches available to correct hyponatremia in these patients are described in the context of recommendations and guidelines recently generated by special groups. IntroductionNoxical studies have demonstrated a significant association between hyponatremia and mortality in patients admitted to hospitals (,) or intensive care units (,). This association is consistent and well recognized in several etiologies and comorbidities, including heart failure (), cirrhosis (), neoplasms (), and CKD (). Hypoatremia has been felt to be a marker of serious and advanced diseases and not a direct contributor to excess mortality (). We review whether the association observed in sick hospitalized patients extends to outpatient patients with mild chronic hyponatremia who have mild or non-smouse symptoms. However, the adaptation of the brain to hyponatremia makes them prone to complications related to morbidity and treatment. We present data on possible results of mild chronic hyponatremia and its treatment that should be weighted against the benefit provided by its correction. Meaning of mild chronic hyponatremia Chronic hyponatremia and mortality risk As part of the baseline evaluation of the Copenhagen Holter Study, Sajadieh and others () measured the concentration of plasma sodium (PNa) in a cohort study aimed at addressing the value of the 48-hour Holter recording in risk assessment of 671 subjects without apparent cardiovascular disease. After adjustment by age, sex, smoking, diabetes, LDL cholesterol and systolic BP, PNaHoorn et al. () PNa base measurement in 5208 subjects in the Rotterdam Study, a prospective cohort designed to evaluate the risk factors for various diseases in the elderly population. With a prevalence of 7.7%, hyponatremia was an independent predictor of mortality, even after adjusting for demographies and comorbidities, with an HR of 1.21 (CI of 95%, 1.03 to 1.43; P=0.02). Gankam-Kengne et al. () analyzed the importance of the PNa baseline in the study of the heart of Dallas with the objective of identifying the biological, ethnic and socio-economic determinants of cardiovascular health differences between 3551 subjects. The prevalence of hyponatremia was 6.3%. After demographic adjustments, major comorbidities and other factors, hyponatremia remained an independent risk factor for mortality, with a RRH of 1.75 (CI of 95%, 1.08-2.81; P=0.02). In a cross-sectional study, Mohan et al. () measured PNa in 14,697 adults who participated in the 1999-2004 National Health and Nutrition Survey (NHANES). In an estimated prevalence of 1.72%, hyponatremia was associated with a death RH of 3.61 (95% CI, 2.31 to 5.63; PTaken together (), data strongly support the view that hyponatremia is associated with a higher risk of mortality in external patients, as it is in those who are hospitalized. Studies that report the association of mild chronic hyponatremia and mortality in outpatient and community environmentsMinimum chronic hypothremia and risk of morbidityNeurocognitive Deficits. The adaptive brain response to hyponatremia involves loss of osmolites, some of which are neurotransmitters (), making the relationship between hyponatremia and the deterioration of the biologically plausible central nervous system. Several excitatory amino acids, such as glutamate, are lost in adaptation to cell inflammation, a process known as decreased regulatory volume (,). Therefore, it is not surprising that neurocognitive deficits are evident, even in seemingly asymptomatic patients, when such changes are proposed specifically for () ().Studies reporting the association of mild chronic hyponatremia and neurocognitive deficit. In a multifaceted study, Renneboog et al. () performed neurocognitive tests in 16 patients with inappropriate antidyuretic hormone secretion syndrome (SIADH), with each of the services as their own control before and after the treatment of hyponatremia. Attention deficits were evaluated by measuring reaction times and error numbers to a series of visual and hearing stimuli presented to patients, who reacted with a simple motor response. When hyponatremics, the average latency and the number of errors were statistically higher, even compared to volunteers after moderate alcohol consumption. The PNa threshold in which the attention deficit increased significantly was 132 mEq/L.In a retrospective case control study, Gosch et al. () administered the Comprehensive Geriatrics Assessment, a standardized tool for detecting functional and cognitive disabilities, 129 older patients with hyponatremia who were subsequently admitted to a geriatric unit and 129 Northmonatromic controls. After a multivariate analysis, patients with mild chronic hyponatremia had significantly worse results in the cognitive and functional tests of the Integral Geriatric Assessment compared to the controls. Gunathilake et al. () evaluated the cognitive function in asymptomatic community residents of the Hunter community study, a prospective population-based cohort study aimed at assessing important health factors for older persons. The cognitive function was greater in individuals with a PNa of 135 mEq/L compared to those with a PNa of 130 mEq/L (95% CI, 1.56-7.79; P=0.01). Gait Disturbances. Another component of the study of Renneboog et al. () assessed the value by measuring the total path (TTW) after a 10-second tandem walk with open eyes on a pressure-sensitive calibrated platform. TTW was significantly longer during hyponatremia compared to TTW when PNa was restored to normal (). The TTW in the hyponatremic group was even longer than that of volunteers after moderate intake of alcohol. Mild chronic hyponatremia is associated with gait alterations. It shows the recorded projection of the gravity center on a calibrated platform sensitive to pressure or total pathway (TTW) in three patients (A-C) after a 10-second tandem walk from right to left with open eyes. The left panel shows TTW during mild chronic hyponatremia, and the right panel shows TTW after hyponatremia correction. Irregular paths of the pressure center were observed in the hyponatremia (sharp). Reprinted reference, with permission. Falls. To assess the importance of gait disturbances, Renneboog et al. () also studied the prevalence of drops in 122 consecutive patients with hyponatremia and 244 combined controls that presented an emergency department for a period of 3 years. Hyponatremia was associated with a higher prevalence of drops (21.3%) compared to the normonatremic controls (5.3%), with an unadjusted probabilities ratio (OR) of 9.45 (CI 95%, 2.64 to 34.09; PStudies reporting the association of mild chronic hyponatremia and falls In another small retrospective study of psychiatric patients, Bun et al. () investigated the association between mild chronic hyponatremia and risk of fall; 91 patients with hyponatremia were paired with 157 Northmonatromic subjects. Using gradual logistic retreat, hyponatremia was associated with a higher risk of fall (OR, 4.38; 95% CI, 1.33-14.46). The study described above by Gunathilake et al. () found not only cognitive deficits, but also, after adjusting for demography and diuretic use, that a decrease in PNa from 135 to 130 mEq/L was associated with an increase of 32% in the risk of fall. Bone fractures. Several studies have found that the volatility of gases associated with hyponatremia, the most likely proximate cause for the high incidence of falls, also increases the risk of fracture (). Studies that report the association of mild chronic hyponatremia and bone fractures Gankam Kengne et al. () analyzed the association between bone fractures and hyponatremia in outpatients. They identified 513 patients with bone fractures and compared them for age and sex with 513 controls. Hypotremia occurs in 13% of the subjects in the fracture cohort, but only in 3.9% of the controls (PSandhu et al. () studied 364 patients who presented a large bone fracture in the emergency room for an 18-month period and coincided with 364 controls; 9.1% of the patients with hyponatremic fractures compared to 4.1% in the fractureless control group (I) In a retrospective case control study, Tolouian et al. () evaluated the prevalence of hyponatremia in 249 patients of age admitted to hip fracture and compared it to the prevalence in 44 joint admitted outpatient controls for hip or knee replacement surgery. The prevalence of hyponatremia in cases and controls was 16.9% and 4.6%, respectively. After controlling the age, hyponatremia was associated with a higher risk of hip fracture (OR, 4.8; 95% CI, 1.06-21.67; P=0.04). More recently, Jamal et al. () studied the association of hyponatremia with fractures between 5122 men of the community of elders who inhabited using data from the Osteoporotic Fractures in Males Estudio. The basal prevalence of hyponatremia was 1,25%. Hypoatremia reported a higher risk of hip fracture (HR, 3.48; 95% CI, 1.76-6.87) as well as a higher risk of prevalent (HR, 2.78; 95% CI, 1.46-5.30) and incident (HR, 3.36; 95% CI, 1.36-8.27) morphometric fractures (i.e., fractures identified by x-ray instead of normal hormone. After adjusting for co-founders, including drops and low DMO, the relationship between hyponatremia and fractures was not reduced. It is of interest that the aforementioned Rotterdam study has found an association between hyponatremia and independent fractures of falls. This is opposed to a primary role for falls, because vertebral fractures, which were also associated with hyponatremia, are usually not caused by trauma. Osteoporosis. Verbalis et al. () have undertaken studies to better define the relationship between hyponatremia and bone metabolism using a SIADH rat model. Hyponatremic rats had a 30% reduction in bone mass compared to fluid-restricted controls that also received demopressin but did not develop hyponatremia. There were no significant differences in serum calcium, parathyroid hormone and urinary excretion of calcium between groups. The microcompputed tomography showed a decrease in bone volume, cortical thickness and the trabecular number in all hyponatremic animals compared to the controls. Hypoatremia increased the number of osteoclasts by bone area compared to the controls, suggesting that the increase in bone resorption, rather than the decrease in bone formation, was the predominant mechanism. In a follow-up study, Barsony et al. () examined the effects of hyponatremia on the number and activity of osteoclast. Exhibition of monocytic and monocytic bone marrow cells taken from hyponatremic rats at low extracellular concentration of sodium, maintaining a normal extracellular osmolality by adding manitol, stimulated directly osteoclastogenesis and osteoclasto activity. These observations have been complemented by the work of Tamma et al. (), which found that the vasopressin V1A receptors and vasopressin V2 receptors (V2R) are present in osteoblasts and osteoclasts of wild mice and that vasopressin injected in these animals stimulates bone resorption by increasing osteoclast activity and inhibiting bone formation by decreasing osteoblast2 activity through stimulation. This last observation suggests that the antidyuretic hormone (ADH) contributes directly to osteoporosis. A cross-sectional study with the NHANES III database that investigated the association between hyponatremia in the general population of 55 years and more and the risk of osteoporosis provides the clinical meaning to the previous observations (). After adjusting for age, sex, BMI, physical activity, level 25(OH) of vitamin D3 and diuretic use, hyponatremia (mean PNa was 133±0.2 mEq/L) was associated with a higher risk of osteoporosis in the femoral neck and total hip, with RWs of 2.87 (9035% CI, 1.41-5,81; P=0.003) and 2.85 % Hyponatremia was associated with a lower MMD and bone mineral content in the total hip and lumbar spine in the unjustified model but lost its meaning when adjusted for sex, age and BMI. However, using multiple regression analysis, a dose-responsive ratio was found between the reduction of PNa and the decrease of the MMD hip, bone mineral content and T-score. In short, the increase in data has accumulated to support the claim that mild chronic hyponatremia, while apparently asymptomatic, is associated with cognitive deficits, gain disorders and falls. These combined with a hyponatremia effect to promote bone loss result in increased risk of fracture (). Management of Chronic Hypotatremia MildDespite the absence of randomized control tests that evaluate the effectiveness of various treatment approaches to mitigate the morbidities described above or the increase in hyponatremia-related mortality, the consensus panels in the United States and Europe have submitted recommendations from experts and clinical practice guidelines, respectively, for the treatment of such patients in various settings (, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , We analyze here the approaches available to treat mild chronic hyponatremia specifically for the outpatient with SIADH (). The main objectives in the treatment of hyponatremia are to limit water intake and promote the excretion of renal water. The latter can be achieved by increasing the load of the urine solute, decreasing the medular osmotic gradient responsible for water reabsorption, or inhibiting actions of ADH (). Mechanism of action of drugs commonly used to treat hyponatremia. (A) ADH works by stimulating the V2 vasopressin receptors (V2Rs) located in the basolateral membrane of the main cells in the collector duct (CD). V2Rs are Gs protein receptors that, when stimulated, increase the production of cAMP by mediated conversion of ATP into cAMP. High levels of cAMP activate protein kinase A (PKA), which in turn phosphorylates store aquaporine 2 (AQP2) that contains vesicles and leads them to the apical membrane of CD cells, increasing water permeability. The transport of NaCl to the medulla through the Na+-K+-2Cl (NKCC2) cotransporter, located in the apical membrane of the cells in the thick ascending extremity of the Henle loop, is essential for the generation of at least half of the maximal medulla concentration gradient (600 mOsm/kg), which constitutes a main driving force along. Loop diuretics work in hyponatremia by inhibiting NKCC2 activity and thus interfering with the generation of hypertonic medulla. Vaptans bind V2R, interfering with ADH action in their receiver. Demeclocycline inhibits the ADC enzyme and, perhaps, also has some post-ADC actions. b) The tubular connection CD and cortical and external medullars are waterproof to the urea. The internal media CD (IMCD) is permeable to urea under the influence of ADH by activating UTA1 and UTA3. Urea works as an osmotic diuretic in the IMCD, and probably along the connecting tubular and CD. In the BMI, the high luminal urea will tend to deregulate the urea conveyors. Also, if the luminal flow rate is high, there will be less time for the transport of urea. ADH, anti-diauretic hormone; CIC-Kb, basolateral chloride channel; ROMK, external renal medullary potassium channel; TALLH, a thick ascending member of the Henle, UTA loop, urea conveyors. Limitation of water intakeDue to the intake of water in excess of the patient's ability to excrete it is central to the physiopathology of hyponatremia, the limitation of water intake presents a cogent option for treatment. As such, it is the first most common step taken by most doctors. Fluid restriction should include all fluids and not just water. However, what degree of fluid restriction is necessary, and this approach will work constantly on each patient? To answer these questions, it is useful to review the normal balance of water, which is represented in . Consequently, the amount of fluid restriction required to achieve a negative balance of water must be lower than the sum of urine and insensitive losses. An alternative thumb rule is to restrict the fluid in an amount that is 500 ml less than the 24-hour urine volume (). Normal water salt A more predictable way of estimating the amount of fluid restriction required to achieve changes in the PNa is provided by the electrolyte-free water cleaning formula (CeH20), which represents the amount of free water excreted by the kidneys for a period of 24 hours: where the CeH20 is electrolyte-free water cleaning, V is the urine volume in 24 hours, UNa is the concentration of urine. If information about V is indisposable, continuous CeH20 and therefore its effect on PNa can be evaluated from a stain urine by calculating the urine ratio to plasma electrolyte [(UNa+UK)/PNa)]. A (UNa+UK)/smart net1 indicates a negative CeH20 (i.e. net free water retention) and anticipates a decrease in PNa. On the contrary, a (UNa+UK)/PNaRecommended fluid-restricting Masters on the basis of the urine ratio to plasma electrolytesSyms with SIADH usually have (UNa+UK)/PNa Confeder1 and therefore a negative CeH20. In such cases, the tolerable restriction of the fluid is likely to result in the improvement of the PNa, and that additional therapies are usually needed. Other predictors of the probable failure of fluid restriction are urine osmolality √500 mOsm/kg, urine volume 24 hours Only a randomized study conducted in children with acute meningitis addressed the effectiveness of fluid restriction. Fluid restriction was effective to increase PNa in patients with hyponatremia but had no advantage in improving results (). In addition, in the data obtained in a recent record of √3000 subjects with hyponatremia, the increase of PNa observed with fluid restriction in the first 24 hours was not significantly different from that observed in nontreated patients (). PNa usually increases slowly and only for 1–2 mEq/L with fluid restriction alone. Fluid restriction is usually poorly tolerated due to an associated increase in thirst. When the fluid restriction fails or is expected to fail, other measures require consideration. Promotion of the Excresion of Renal WaterIncrease of the charge of Soluto de Urine. The excretion of the urine solute is a determinant of the excretion of free water (). NaCl works in hyponatremia in part increasing the load of the urine solute, causing an electrolytic diuresis. However, NaCl is used in conjunction with diuretics of loop to treat hyponatremia, where its main role is the restoration of urinary losses of sodium and the prevention of negative sodium balance (,). There are no trials that evaluate therapy with NaCl alone, and the few reported cases that use it are combined with diuretics of loop. NaCl is available as 1-g tablets (17 mEq sodium and 17 mEq chloride). The usual doses for NaCl tablets are 6 to 9 g per day in divided doses (e.g. 2 to 3 g twice or 3 times a day). Recycling urea and its reabsorption in the internal medular collection duct (IMCD) by UTA1 and UTA3 carriers play an important role in the fine adjustment of renal water reabsorption (,). However, urea is an ineffective solute; when its excretion rate increases (e.g., urea tablets, high protein diet, post-ATN diuresis, or post-obstructive diuresis), urea cannot be absorbed fast enough to balance between tubular lumen and intracellular space of conduit cell collection (CD). In such circumstances, the urea becomes an authentic solute that compels the excretion of water (). Urea works in hyponatremia inducing osmotic diuresis and decreasing the reabsorption of free water in BMI () and probably along the connecting tub and CD (). In an animal model, urea improved hyponatremia in SIADH by also reducing compensatory natriuresis that contributes to hyponatremia in this syndrome (). The only clinical evidence for urea effectiveness in the treatment of hyponatremia comes from case series (–). Decaux et al. () reported seven patients with chronic SIADH diagnosis who could not tolerate strict fluid restriction and were treated with oral urea 30 or 60 g/d. Despite the normal intake of water, urea corrected hyponatremia in the seven patients (average PNas treatment and during the treatment were 115.6±6 and 136±3.5 mEq/L, respectively), with those with the highest intake of fluid requiring higher urea doses (60 g/d). Although PNa rose significantly with urea treatment, concentrations fluctuated widely, and this variation was related to fluctuations in the daily intake of water. No significant side effects were observed after up to 270 days of treatment. Soupart et al. () also reported the use of urea in a number of cases of 13 patients with chronic hyponatremia of SIADH. PNa increased from an average of 125±3 to 135±3 mEq/L to 1 year with the use of vaptans. The vaptans were then discontinued, allowing the recurrence of hyponatremia. Urea was initiated for an additional 1 year, at the end of which PNa was again 135±2 mEq/L. Urea was well tolerated, and no significant adverse events were reported. Current European guidelines favor their use as a second-line therapy (after fluid restriction) on the use of vaptans for the treatment of SIADH (). However, there is no Urea pharmacopea formulation, and it is not approved for this use by the Food and Drug Administration (FDA). The recommended doses are 30 to 60 g per day in divided doses (). Urea has many advantages: it acts immediately and has minimal toxic effects, even in plasma concentrations of 193–301 mg/dl. If the osmolality of the urine is high and the kidney function is well preserved, the furosemide is preferred over the urea, because it will take a high dose of urea to produce enough osmotic diuresis to be effective (,). Urea has been found especially effective in the treatment of nephrogenic syndrome of inappropriate antidyuresis, a genetic disorder caused by activating mutations in V2R, where vaptans are ineffective (). The BUN and the osmolality of the urine are expected to increase with the urea. Urea has a bitter taste, which limits its use, but combines it with sweet substances, such as orange juice, can relieve this problem (,). Decrease the Medular Osmotic Gradient. The main driver for water reabsorption in the CD is the osmotic gradient generated by the renal medulla, which has tonicity of 1200 mOsm/kg at the level of the papylla. In the internal medulla, NaCl contributes to about 50% of this hypertonic medulla, with urea contributing to the other 50%. The first step in transporting NaCl to medulla is through the Na-K+-2Cl cotransporter - located in the apical membrane of the thick ascending extremity of the loop of the Henle cells. The diuretics of loop inhibit this conveyor, reducing NaCl delivered to the medulla and thus diminishing the medulla medular osmotic gradient necessary for water reabsorption on the CD and thus increasing the excretion of free water (,). The only clinical evidence for the effectiveness of loop diuretics in the treatment of hyponatremia comes from case series, and all in combination with NaCl tablets (,,,,). It should be noted that most patients in these cases reported and case series improved their PNa with the combination of diuretics of loop and NaCl tablets, despite a relatively normal intake of fluid. Although rare, reports of hyponatremia have also been reported in association with the use of diuretic loop (,). The dose of furosemide is 20–40 mg PO once a day. Act of loop diuretics immediately. They are not approved by the FDA to treat hyponatremia. Inhibite ADH actions in the Kidney. Some causes of SIADH (e.g. neoplasms and idiopathic) are not easily reversible. In such cases, agents that antagonize the renal action of ADH should be considered: antagonists of demeclocycline or vasopressin receptors. Demeclocycline, a tetracycline derivative, decreases the activity of adenylcyclase and consequently, the synthesis of cAMP (,) and the abundance of aquaporin 2 in BMI (), resulting in a reversible form of nephrogenic insipidus. The series of cases reported modest demeclocycline effects on the improvement of PNa in patients with hyponatremia (). However, the only clinical trial in existence is a cross-sectional study of double-blind placebo with nine psychiatric patients with episodic or chronic hyponatremia caused by primary polydypsy (). Researchers found no significant difference in the number of episodes of hyponatremia during the drug administration period compared to the placebo period. However, demeclocycline is used in refractory cases of hyponatremia. The proper dosage of demeclocycline is 600–1200 mg/d in divided doses (). The start of the action is usually 3 to 4 days (). Demeclocycline is not approved by the FDA to treat hyponatremia. The use of demeclocycline has been associated with severe adverse reactions, such as skin photosensitivity, the risk of superinfection and nephrotoxicity, especially in patients with cirrhosis (). Demeclocycline nephrotoxicity seems to depend on the dose, requiring a slow dose of titration and monitoring of kidney function. Given the concern about severe side effects, the guidelines of European clinical practice on the diagnosis and treatment of hyponatremia recommend against their use (). Vaptans point directly to the hyponatremia mechanism in the high states of ADH competing with ADH for the union in the V2R on the CD. Tolvaptan is the only oral vaptan approved by the FDA for use in outpatient treatment of euvovolemic or hypervolemic hyponatremia. The ability of the vaptans to increase PNa is widely documented. In fact, vaptans are the only interventions for the treatment of hyponatremia for which there are randomized control tests (i.e., SALT1 and SALT2) () complemented by two well conceived metaanalysis (,). However, there is a risk of partiality of publication, since most of the vaptans trials, except for SALT trials, were performed in a relatively small number of patients, and almost all were sponsored by the industry. In addition, there is almost a complete lack of head-to-head trials that compare vaptans to other therapies used. To avoid excessive correction, vaptans should be started and restarted as patients with frequent monitoring of PNa. Tolvaptan starts at a dose of 15 mg daily. It can be increased to 30 mg after 24 hours and then 60 mg after 24 hours. To mitigate the rate of increase in PNa, patients should not be restricted by the liquid for the first 24 hours. The long-term administration of up to 4 years suggests the maintenance of effectiveness (). Several limitations must be considered in the use of vaptans. As with urea and demeclocycline, vaptans are contraindicated in hyponatremia and are not indicated in patients with severe neurological symptoms, such as seizures, because they have not been tested in such subjects and the beginning of changes in PNa is not fast enough (at least 4-8 hours) to quickly address symptoms. Vaptans are metabolized by CYP3A4, and therefore, precaution should be exercised when co-administered with CYP3A4 inhibitors (e.g. ketoconazole) or inducers (e.g., rifampin), which increase or decrease drug levels, respectively. More recently, concerns about liver toxicity have emerged. The TEMPO 3:4 study designed to determine the effectiveness and safety of the hopperptan in the treatment of autosomal dominant polycytic kidney disease () reported an increase in the tests of liver function in the Tolvaptan group compared to the placebo group. It should be mentioned that the dose of tolvaptan used in this study was four times the dose used in hyponatremia tests, in which such toxicity was not observed. The FDA recommends not to use hoppers in patients with liver disease or for a period of 30 days. The development of osmotic demylation syndrome (ODS) is always a concern when hyponatremia is corrected. Although PNa reached the hypernatremic range in some patients involved in the above-mentioned trials, the SDG was not reported in any of them. Since then, in total, 12 patients with ADHD have been reported in association with alvaroptan. Only two of these cases have been published (S.A.A. Harb and C. Alraies, unpublished data) (). However, some other factors might have contributed to PNa correction in published cases. In the first case, lvaptan continued for 4 days, despite an initial increase of PNa from 126 to 142 mEq/L, with an additional correction of 181 mEq/L per day 4 when it finally stopped tolvaptan. In the second case, the use of hopperptan was in close temporary relationship with hypertonic saline use. The other 10 unpublished cases have been reported to the FDA (). These adverse events generated a warning letter from the producer company (). Lack of response to vaptans may occur in some settings (). These include the presence of very high levels of circulating ADH, a diluted vasopressin defect (low distal birth as a result of the decrease in GFR and proximal tubular reabsorption such as advanced heart failure or cirrhosis), excessive intake of water, and nephrogenic syndrome of inappropriate anti-diuresis (). Despite the well-established effects to increase PNa, there is no data to determine whether vaptans affect the mortality described or alter the risk of various morbidities associated with hyponatremia. There is also uncertainty as to whether vaptans reduce the use of health resources by affecting hospitalization rates and the duration of the stay. In this direction there was a statistically insignificant tendency in an analysis of the EVEREST judgment () and a significant effect on the SIADH subgroup in a post hoc analysis of the SALT judgments (). However, for the average patient, the cost of vaptans remains an impediment to their use (). The lack of mortality and morbidity are accompanied by concerns about efficacy and safety led the European practice guidelines committee not to recommend the use of varitans in euvolemic hyponatremia and even to recommend against its use in hypervolumemic hyponatremia (). This contrasts with the recommendations of a group of experts who consider the use of vaptans to be a reasonable option in both contexts (). It should be noted that the latter group was supported by the financing of Otsuka America Pharmaceuticals Inc., the manufacturer of hopperptan, and that a substantial proportion of panel members also had funding from Otsuka America Pharmaceuticals Inc.Conclusions Mild chronic hyponatremia is not benign as previously thought and can contribute directly to increased morbidity and possibly mortality (,). Although some of the previous pathologies are clearly related to hyponatremia, whether treating the disorder will reverse this sequence of events is not yet known. We are of the opinion that patients with mild chronic hyponatremia associated with unstable gait, recurrent unexplained falls, a high risk of fracture, or severe osteoporosis could benefit from treatment. The benefits against risks are likely to shift in favor of long-term outpatient use of hoppers when fluid restriction and all other therapies have failed. We recommend that future studies address the following topics: (1) the effectiveness, safety and tolerability of urea in the treatment of hyponatremia; (2) the effectiveness and safety of vaptans compared to other therapies; and (3) the effects of vaptans and other therapies on the significant results of the patient, such as falls and fractures. DisclosuresH.R.B. does not receive financial support. T.B. was in the office of the speaker of Otsuka America Pharmaceuticals Inc. He was also the principal investigator of the SALTWATER trial, a trial sponsored by Otsuka America Pharmaceuticals Inc. T.B. currently has no relationship with Otsuka America Pharmaceuticals Inc. It does not receive payments, does not possess actions, and does not have other conflicts of interest. FootnotesPublished online ahead of printing. Date of publication available in .ReferencesIn this issueDating Manager ProgramsAccess to the More section in this section... Related articlesKeywordsArticles Information for authorsAboutJournal InformationMore info© 2021 American Society of NephrologyPrint ISSN - 1555-9041 Online ISSN - 1555-905XX

Toppin Salt - 100 Tablets | Amcal

Urea for the Treatment of Hyponatremia | American Society of Nephrology

Salt Tablets for Runners: Everything You Need to Know | Rockay

Salt Tablets - When to Take Them & Where to Buy Them | Livingit | Electrolytes, Heat stress, Salt

Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia - American Family Physician

Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia - American Family Physician
Management of hyponatraemia

Hyponatremia Treatment Algorithm CHF, Cirrhosis ... | GrepMed
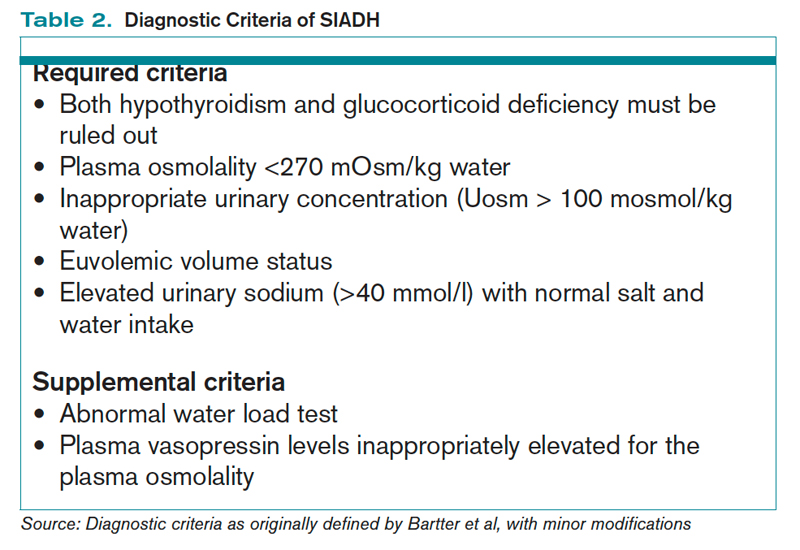
How Is SIADH Diagnosed and Managed? | The Hospitalist

Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia - American Family Physician

Hyponatremia - EMCrit Project
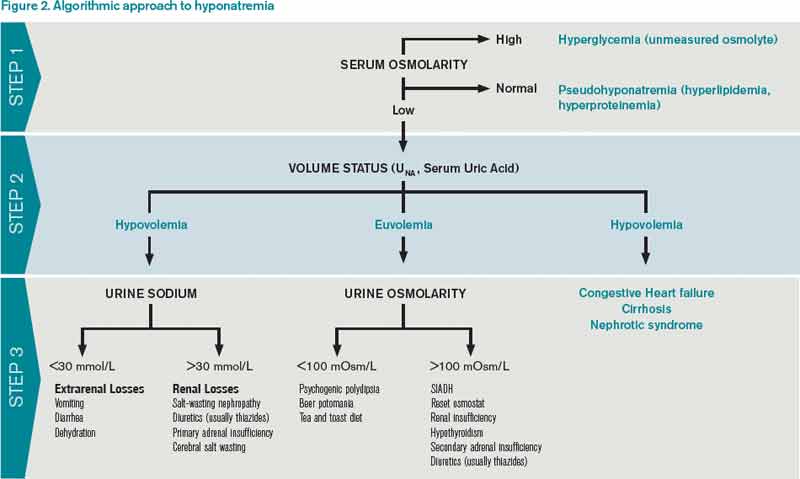
How Should Hyponatremia Be Evaluated and Managed? | The Hospitalist
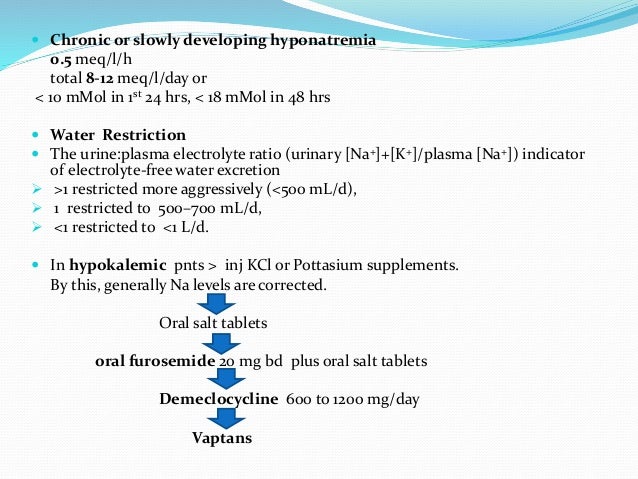
Approach to hyponatremia

Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia - American Family Physician

What are Salt Pills? (with pictures)

Salt Tablets for Hyponatremia (Page 1) - Line.17QQ.com

Efficacy of Furosemide, Oral Sodium Chloride, and Fluid Restriction for Treatment of Syndrome of Inappropriate Antidiuresis (SIAD): An Open-label Randomized Controlled Study (The EFFUSE-FLUID Trial) - American Journal of Kidney Diseases

Amazon.com: Sodium Chloride Tablets 500 mg (0.5 Gram), USP Normal Salt Tablets - 200 Tablets by CitraGen Pharmaceuticals, Inc.: Health & Personal Care

Salt Tablets for Hyponatremia (Page 1) - Line.17QQ.com
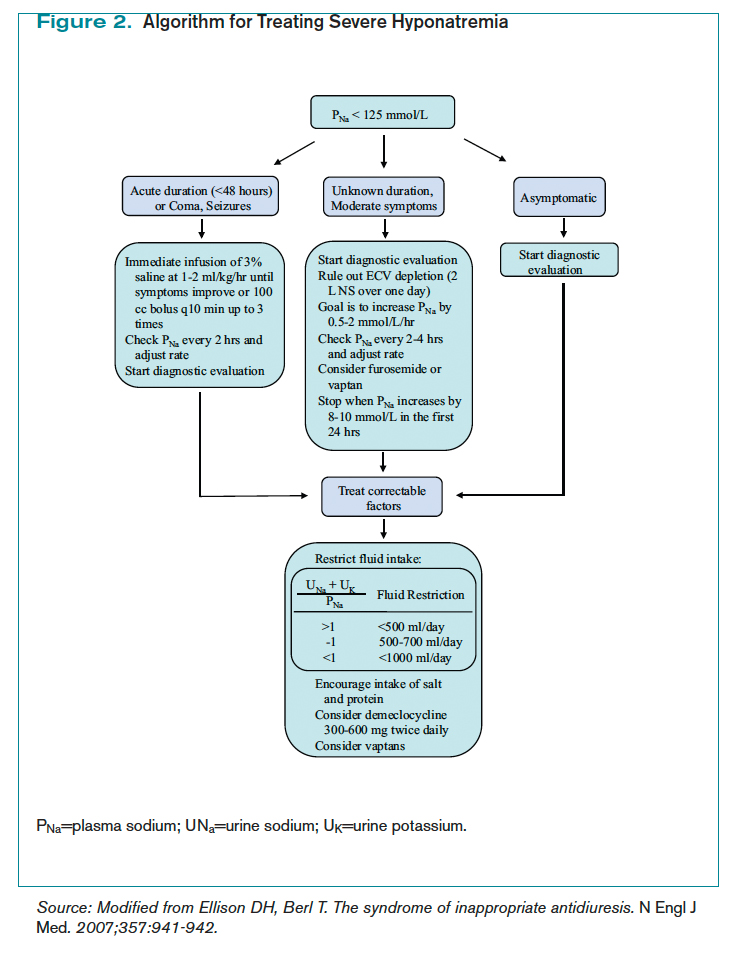
How Is SIADH Diagnosed and Managed? | The Hospitalist

My first Tweetorial: When salt tablets work in hyponatremia (and when they do not) – Precious Bodily Fluids

HYPONATREMIA AND THE EMERGING ROLE OF TOLVAPTAN IN CARDIOLOGY - ppt download

How Is SIADH Diagnosed and Managed? | The Hospitalist

Hyponatremia - EMCrit Project

HYPONATREMIA - UK

PDF) Hourly oral sodium chloride for the rapid and predictable treatment of hyponatremia

Mild Chronic Hyponatremia in the Ambulatory Setting: Significance and Management | American Society of Nephrology

Hyponatremia - YouTube
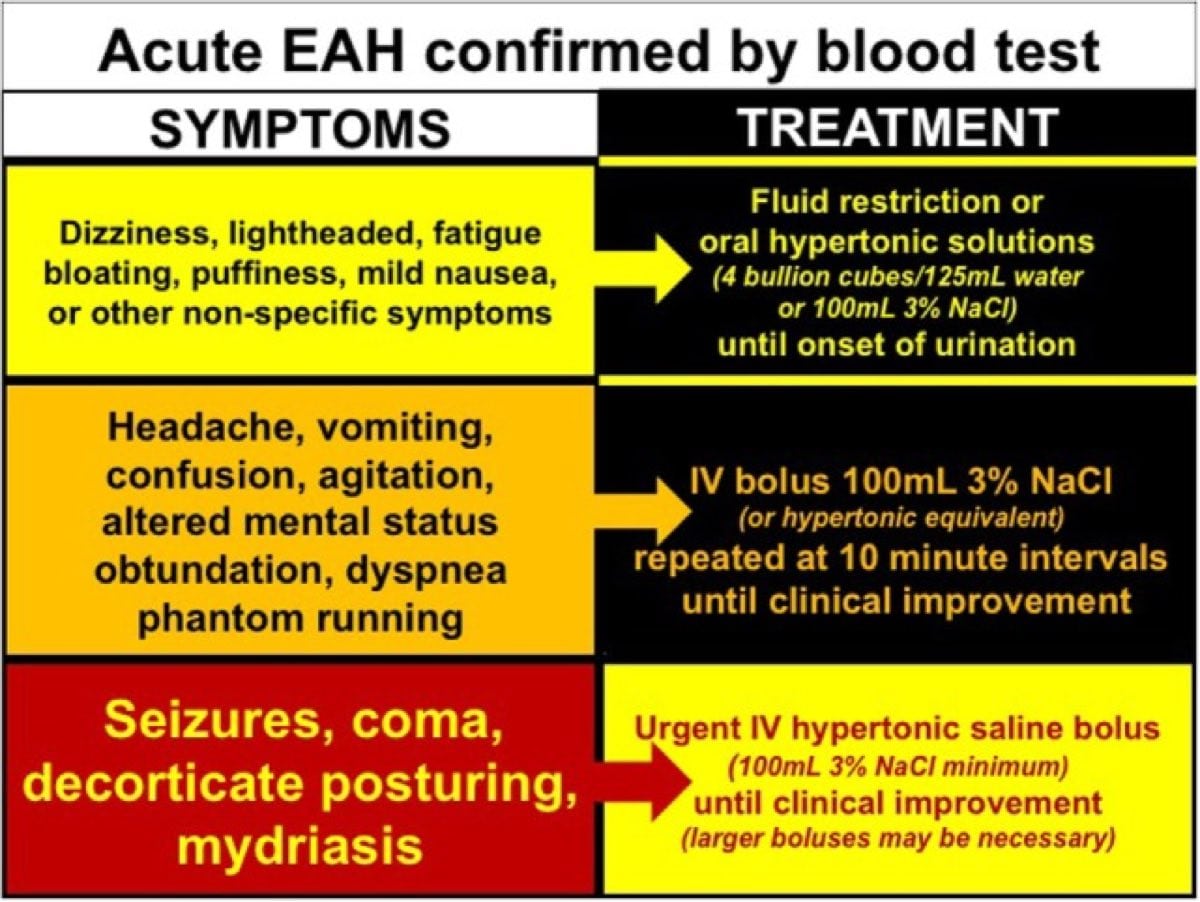
Exercise-Associated Hyponatremia: The (Not So) Salty Truth - iRunFar

Diagnosis and management of hyponatraemia in hospitalised patients - Reddy - 2009 - International Journal of Clinical Practice - Wiley Online Library
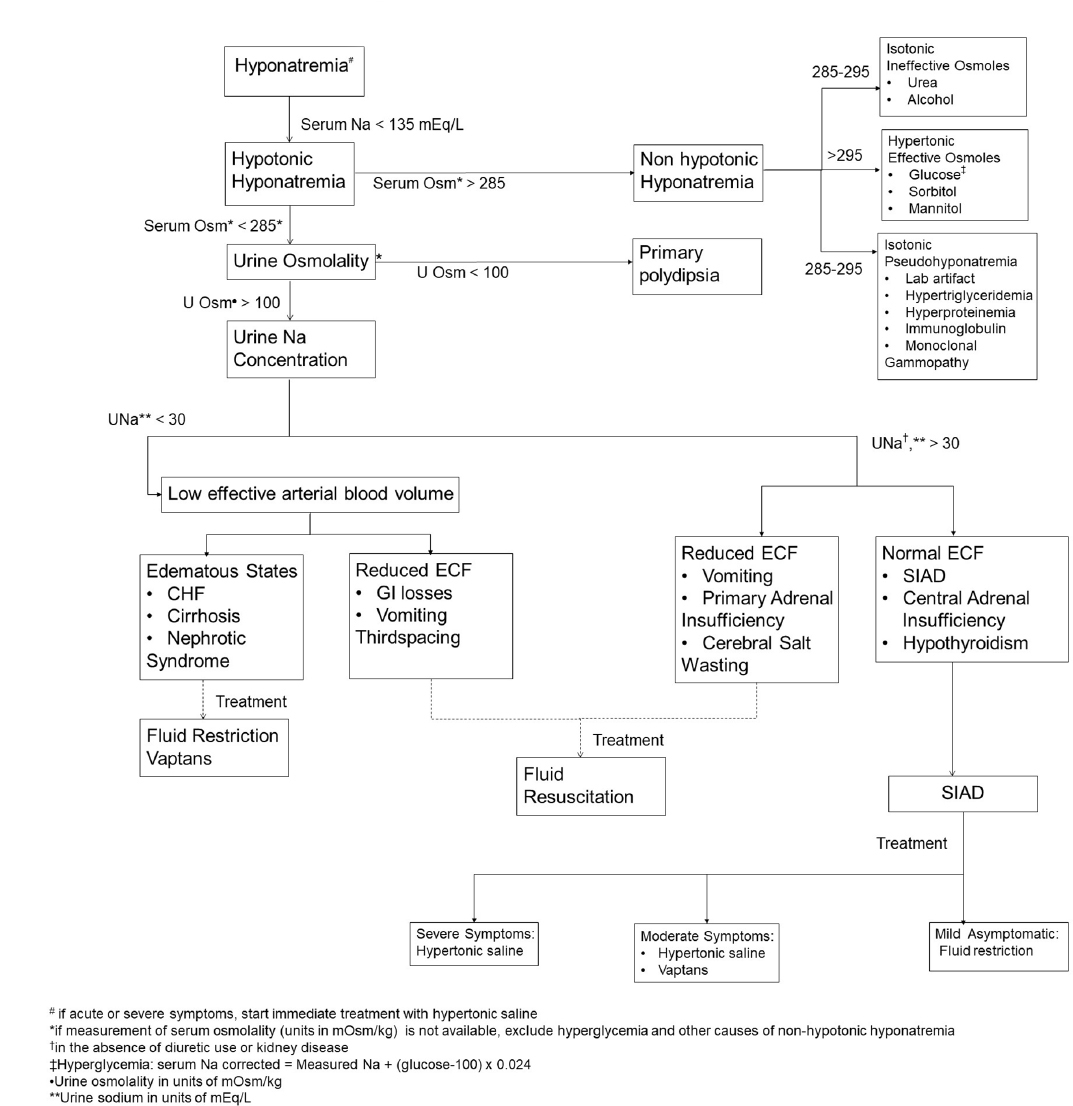
Recent advances in the management of hyponatremia in cancer patients

NephMadness 2018: Hyponatremia Region – AJKD Blog

Rapidity of Correction of Hyponatremia Due to Syndrome of Inappropriate Secretion of Antidiuretic Hormone Following Tolvaptan - American Journal of Kidney Diseases

32: Why normal saline makes hyponatremia worse in SIADH - Pharmacy Joe -

Salt Tablets for Effective Hydration | RunnerClick | Running hydration, Hydration, Tablet
Edgar V. Lerma 🇵🇭 on Twitter: "The use of salt tablets in the treatment of euvolemic hyponatremia is asso. w/ a small but significant improvement in serum Na (even after adjusting for

Hyponatremia in SIADH: Role of the Vaptans - ppt download

Hyponatremia and the Brain - ScienceDirect

Hyponatremia in the cancer patient - Kidney International

Salt Tablets for Runners: Everything You Need to Know | Rockay
Posting Komentar untuk "salt tablets for hyponatremia"